
IHC testing—using CLDN18 antibodies to detect CLDN18.2 in G/GEJ tumour samples
CLDN18 antibodies can identify both CLDN18 isoforms—CLDN18.1 and CLDN18.2; however, when evaluating G/GEJ tumour tissue, staining can be attributed to the presence of CLDN18.2 only, because3,4:
CLDN18.1 is the dominant isoform expressed in normal and malignant lung tissue, and its expression is negligible or absent in G/GEJ cancers3,4
CLDN18.2 is normally expressed in gastric epithelium and expression is often retained in malignant gastric tissue3,4
CLDN18 antibodies (such as 43-14A clones) are appropriate to measure CLDN18.2 expression in G/GEJ3,5
Sample preparation and preanalytics
Appropriate specimen handling and preparation are essential to ensure the accuracy of biomarker results.1
Sample preparation
Guidelines recommend daily tissue processor maintenance per the manufacturer recommendations and rigorous quality maintenance of processor fluids, including formalin pH/purity and water contamination of alcohols.1
Cold ischemic time
Cold ischemic time should be limited to ≤60 minutes according to current guidelines.1
Fixation
Guidelines provide recommendations regarding dimensions and duration involved in sample fixation.1
Key dimensions1
Tissue should be completely submerged in fixative
Ensure a fixative volume to tissue mass ratio of no less than 4:1, with an optimal ratio of 10:1
Paraffin should be melted at <60°C
Specimen should contain sufficient tumour tissue for analysis
Key durations1
As part of stabilisation, tissue should be fixed in 10% neutral phosphate-buffered formalin (pH 7.0) for at least 6 hours and no longer than 24 to 36 hours
If the tissue has high fat content, fixation may require up to 48 hours
Suboptimal tissue fixation1
It is important to optimize preanalytical variables to minimize staining artifacts.
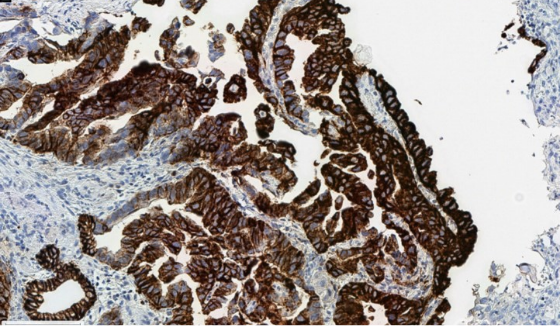
Cytoplasmic blushing due to suboptimal fixation, which can interfere with accurate membranous scoring.
Specimen preparation2
Routinely processed, formalin-fixed, paraffin-embedded (FFPE) tissues are suitable for use with IHC testing
Specimens that are fine-needle aspirate (FNA), cytology specimens, or metastatic bone lesion do not qualify for CLDN18 staining
Tissue sections can be cut at 3 µm-6 µm*
Before staining, the cut slides should be dried completely either at room temperature (air dried)
or by offline baking (baked in oven) at 60°C for 60 minutes*
*CLDN18-specific.
Storage conditions1
To ensure the integrity of nucleic acid and protein in blood/serum, the conditions must be:
Dry
Pest-free
Room temperature (18°C to 25°C)
Testing
Antibody options for evaluating CLDN18.2 expression via IHC
A number of assays and antibodies are available to assess CLDN18.2 expression. The list below is not exhaustive. An appropriate, validated IHC antibody/assay with high performance should be used when guiding clinical decision-making.
| Name | Company | Clone Name | Clonality and Host | Use |
|---|---|---|---|---|
| VENTANA® CLDN18 (43-14A) RxDx Assay | Ventana Medical Systems, a member of the Roche Group | 43-14A | Mouse, monoclonal | IVD |
| Recombinant Anti-Claudin 18 antibody (43-14A) | Abcam | 43-14A | Mouse, monoclonal | RUO |
| Amoy Dx CLDN18 (43-14A) | Amoy Diagnostics | 43-14A | Mouse, monoclonal | RUO |
| LBP Dx CLDN18 (43-14A) | LBP Diagnostics | 43-14A | Mouse, monoclonal | RUO |
| PathPlus™ CLDN18/Claudin 18 Antibody | LSBio, an Absolute Biotech Company | LS-B16145 | Mouse, monoclonal | RUO |
Select appropriate controls
Appropriate controls are essential for the detection of CLDN18.2 in G/GEJ tumour samples. Here are some key points on their selection and use.2,5
Validation controls
Guidelines recommend that laboratories validate and/or verify immunohistochemical tests before placing them into clinical service. These tests should include positive, negative, and borderline tissue, reflecting the intended use of the assay.6
Tissue controls are commercially available through various providers.
Run controls
- It is recommended to use optimal run controls, including positives and negatives2
- Gastric intestinal metaplasia tissue is an example of an appropriate positive and negative control as it demonstrates2:
- Strong membranous staining in normal gastric epithelial cells
- Weak-to-moderate membranous staining of epithelial cells in areas of metaplasia
- Absence of staining in lamina propria, lymphocytes, smooth muscle, blood vessels, and peripheral nerve
- If the positive controls fail to demonstrate staining, results of the test specimen should be considered invalid2
- Known positive tissue controls should be utilised only for monitoring performance of reagents and instruments, not as an aid in determining the specific diagnosis of test samples2
CI=confidence interval. CLDN=claudin. CLDN18.1=claudin 18 isoform 1. CLDN18.2=claudin 18 isoform 2. ESMO=European Society for Medical Oncology. GC=gastric cancer. G/GEJ=gastric/gastroesophageal junction. HER2=human epidermal growth factor receptor 2. IHC=immunohistochemistry. IVD=in vitro diagnostic. OS=overall survival. RUO=research use only receptor 2.
Proficiency evaluations have identified variability in antibody and platform performance
Global concordance study
In the global concordance study, which assessed the reproducibility and comparability of three CLDN18 antibodies—VENTANA (43-14A), LSBio (LS-B16145), and Novus (NBP2-32002)—and IHC staining platforms across a cohort of 27 global laboratories, sensitivity, specificity, accuracy, and precision were analyzed.5,*,†
- Analytical performance of the VENTANA assay was >95% and reproducible across the 27 laboratories vs consensus reference results
- Analytical performance was equivalent to the VENTANA (43-14A) IVD assay for the LSBio antibody when stained on the Dako or Leica platform; staining was also reproducible for the LSBio antibody
- Performance was least consistent for the Novus antibody compared to the VENTANA assay
- Staining was reproducible for the LSBio antibody4
*Antibodies in the study comprised the VENTANA CLDN18 (43-14A) IVD assay from Roche Tissue Diagnostics, the PathPlus™ CLDN18 Antibody from LSBio, and the Claudin-18 Antibody from Novus Biologicals. Platforms comprised BenchMark ULTRA, Dako Autostainer, and Leica Bond.4
†Consensus reference scores from all antibodies for each sample were determined by central pathology review. CLDN18.2 positivity was defined with a threshold of ≥75% of tumour cells expressing membranous CLDN18 with moderate-to-strong (≥2+) staining intensity. Accordingly, participating pathologists were required to submit a binary positive/negative call as well as an estimation of the percent of cells stained. Laboratory-submitted IHC scores were compared to the reference consensus score and considered discordant if the positive/negative binary result differed. Statistical analysis was performed for comparison, and an acceptance criteria of 85% (≥0.85) was applied.4
2024 QuIP independent proficiency testing study7*
Results from the 2024 Quality in Pathology (QuIP) independent proficiency testing study highlight the importance of antibody selection for accurate results.
- Fifty-three† pathology labs participated to assess CLDN18.2 testing proficiency in GC using 10 antibodies
Performance Overview
- A total of 42 participating institutes (~79.2%) successfully passed the independent proficiency test‡
- Among the CLDN18-specific antibodies, the CLDN18 (43-14A) clone was the most frequently utilized and demonstrated consistent performance, with no significant technical or interpretive issues reported
- In the evaluation of CLDN18.2-specific antibodies, significant issues were identified with staining quality; two antibody clones were found to account for 75% of the unsuccessful submissions:
- EPR19202 clone: Achieved a 0% success rate (0/5) due to low sensitivity and weak staining intensity
- ZR451 clone: Achieved a 56% success rate (5/9) but exhibited low staining specificity
Key Considerations
- Accurate determination of CLDN18.2 status relies on the use of well-optimized staining protocols and high-performing antibody clones
- To ensure reliable patient test results, it is essential to maintain ongoing quality control through external quality assessments (EQAs), regardless of the antibody clone employed
QuIP is an accredited and experienced independent institution that specialises in conducting EQAs for IHC and molecular biomarker testing on tissue samples.
*QuIP independent proficiency testing study sponsored by Astellas.
†Fifty-four institutes registered for the open proficiency test and one resigned.
‡Successful passing was defined as ≥ 90% of cases evaluated correctly.
NordiQC External Quality Assessment Results*
The objective of the NordiQC EQA is to evaluate the analytical accuracy of IHC assays for detecting CLDN18.2. Seventy-five laboratories participated in this first assessment with ~87% (65/75) achieving a sufficient mark.8
- 100% of IHC stains submitted using the CE-IVD VENTANA CLDN18 (43-14A) assay were marked as “sufficient,” of which ~89% (8/9) were “optimal”
- For the assessment of 6 concentrated antibodies
- Only ~46% (6/13) of IHC stains submitted were marked as “sufficient”
- The laboratory-developed test utilizing the monoclonal antibody clone 43-14A was the most successful, with 80% (4/5) marked as “sufficient”
NordiQC is a professional scientific organization that conducts EQAs for immunohistochemistry proficiency.9
*NordiQC EQA sponsored by Astellas.
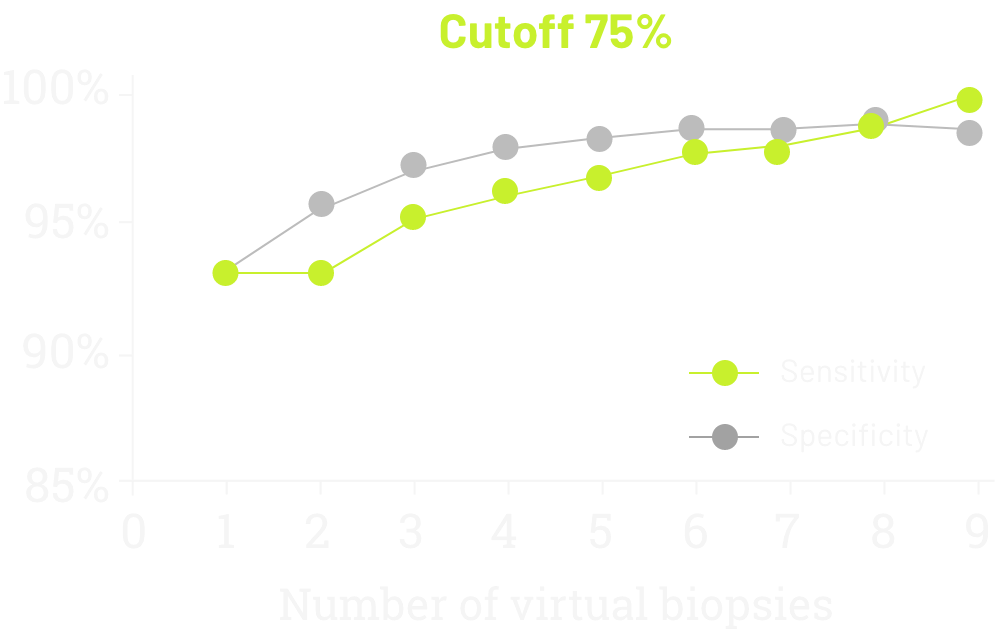
Navigating intratumoural heterogenity
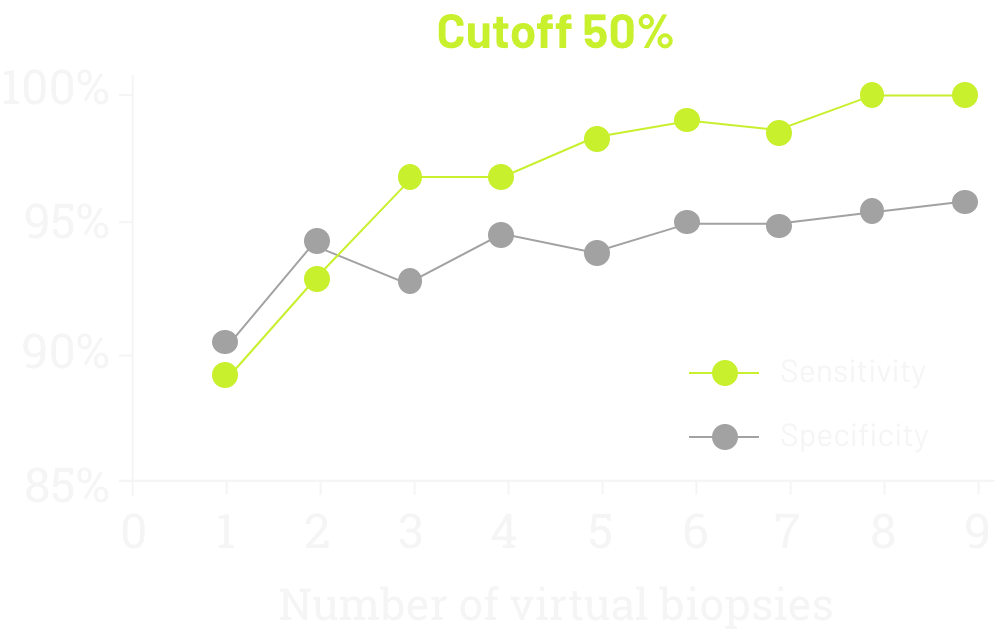
A single-institution study in Italian patients with G/GEJ adenocarcinoma evaluated the sensitivity and specificity of CLDN18 testing according to the number of virtual biopsies scored on 93 surgically treated cases (77 GC and 16 GEC), evaluated by two blinded pathologists.10
- Sensitivity increased steadily upon evaluating additional biopsies
- After 6 biopsies, the increase was minimal
Reflexive testing may improve patient outcomes
Delaying biomarker testing may worsen clinical outcomes
Guidelines recommend testing for all available, actionable biomarkers, such as HER2, at time of diagnosis if metastatic GC is documented or suspected.11
ESMO guidelines recommend testing for CLDN18.2.11
Any new biomarker should be tested concurrently with other biomarkers to allow for timely reporting of results.11,12
Pathologists and oncologists recognize the positive impact of reflexive testing on improved patient care
A recent study surveyed pathologists and oncologists on the importance of biomarker testing immediately following G/GEJ adenocarcinoma diagnosis (reflexive testing).13
- Participants agreed that reflex testing has the potential to improve the speed of treatment decisions and improve patient care
Reflexive testing allows targeted therapy to begin sooner, improving clinical outcomes
In a cohort of advanced cancer patients comprising diverse cancer types (N=1423), implementation of a reflex testing protocol at time of diagnosis was associated with improved overall survival.12
OS at 12 months
We’ve seen evidence that reflexive testing improves patient outcomes. It’s critical that pathologists advocate for testing at diagnosis for all prevalent, actionable biomarkers.
Matteo Fassan, MD, PhD


References: 1. Compton CC, Robb JA, Anderson MW, et al. Preanalytics and precision pathology: pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch Pathol Lab Med. 2019;143(11):1346-1363. 2. Ventana CLDN18 (43-14A) assay [package insert]. Mannheim, Germany: Roche Diagnostics GmbH. 3. Fassan M, Kuwata T, Matkowskyj KA, et al. Claudin-18.2 immunohistochemical evaluation in gastric and gastroesophageal junction adenocarcinomas to direct targeted therapy: a practical approach. Mod Pathol. 2024;37(11):100589. 4. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624-7634. 5. Jasani B, Taniere P, Schildhaus HU, et al. Global Ring Study to investigate the comparability of total assay performance of commercial claudin 18 antibodies for evaluation in gastric cancer. Lab Invest. 2024;104(1):100284. 6. Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138(11):1432-1443. 7. Röcken C, Höhn AK, Neumann J, et al. Concordance of laboratory assays for claudin 18.2 in gastric cancer tissue samples: independent proficiency testing and a descriptive non-interventional study. Virchows Arch. Published online June 28, 2025. doi:10.1007/s00428-025-04138-x 8. NordiQC. Assessment Run C17 2025 Claudin 18.2 (CLDN18.2). https://w.nordiqc.org/downloads/assessments/206_123.pdf Accessed June 16, 2025. 9. NordiQC. About NordiQC. https://www.nordiqc.org/about.php Accessed July 21, 2025. 10. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med. 2021;11(11):1095. Published 2021 Oct 26. 11. ESMO GC Living Guidelines. Version 1.4. September 2024. https://www.esmo.org/guidelines/living-guidelines/esmo-living-guideline-gastric-cancer/diagnosis-pathology-and-molecular-biology Accessed June 16, 2025. 12. Piening B, Bapat B, Weerasinghe RK, et al. Improved outcomes from reflex comprehensive genomic profiling-guided precision therapeutic selection across a major US healthcare system [Abstract 6622]. J Clin Oncol. 2023;41(Suppl 16):6622. 13. West NP, Mansoor W, Taniere P, et al. Best-practice biomarker testing of oesophago-GC in the UK: expert consensus recommendations developed using a modified Delphi. Clin Oncol (R Coll Radiol). 2024;36(11):701-709.
