In G/GEJ tumours, assessment for CLDN18.2-positivity is determined by the percentage of tumour cells stained with moderate-to-strong membrane stain intensity1,4
In G/GEJ tumours, assessment for CLDN18.2-positivity is determined by the percentage of tumour cells stained with moderate-to-strong membrane stain intensity1,4
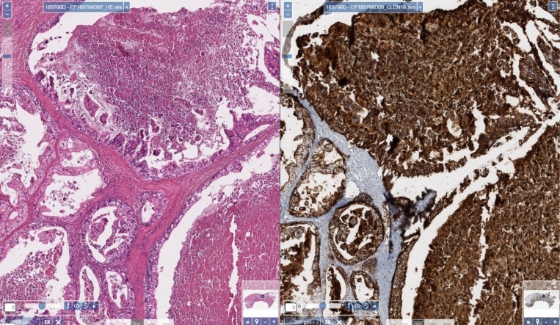
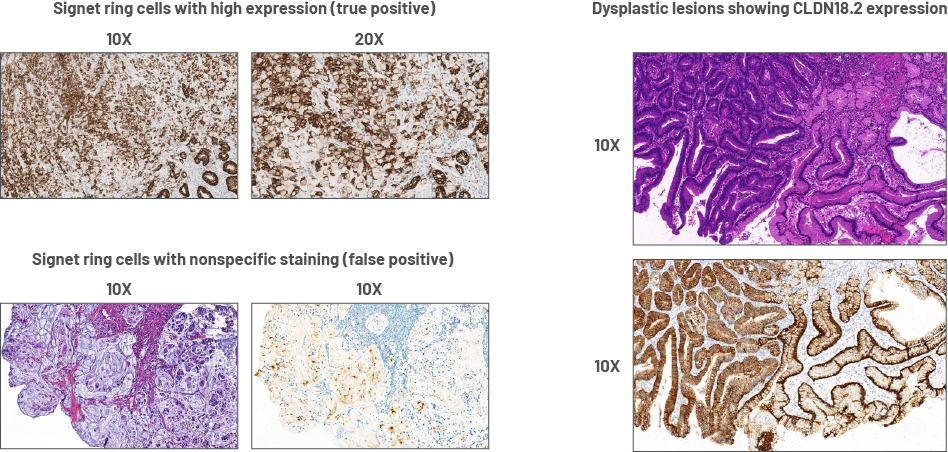
Signet ring cells and dysplastic lesions
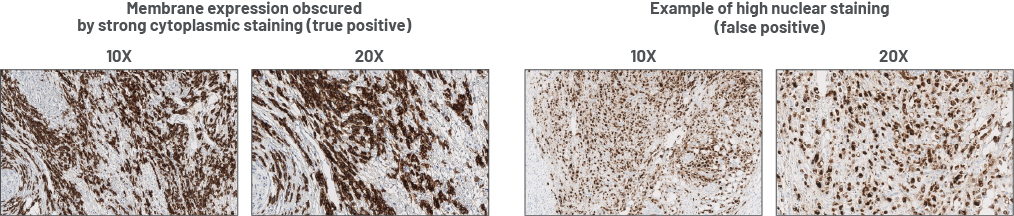
Cytoplasmic vs nuclear staining
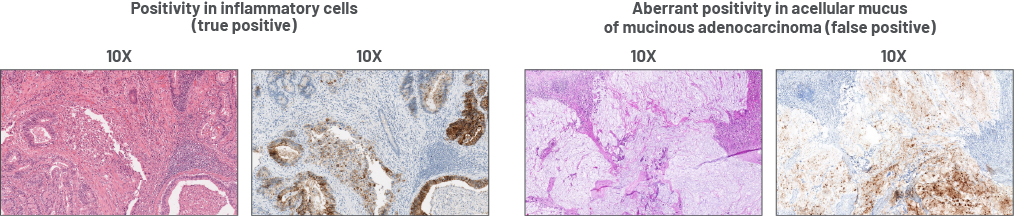
Aberrant positivity
Preanalytical artifacts
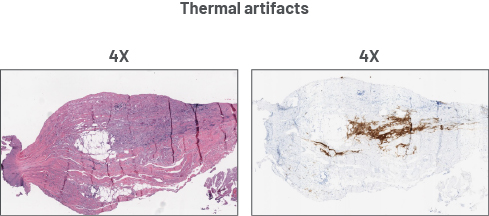
CLDN=claudin. CLDN18.2=claudin 18 isoform 2. H&E=hematoxylin and eosin. HER2=human epidermal growth factor receptor 2. IHC=immunohistochemistry. PD-L1=programmed death-ligand 1.
References: 1. Fassan M, Kuwata T, Matkowskyj KA, et al. Claudin-18.2 immunohistochemical evaluation in gastric and gastroesophageal junction adenocarcinomas to direct targeted therapy: a practical approach. Mod Pathol. 2024;37(11):100589. 2. Shitara K, Xu RH, Ajani JA, et al. Global prevalence of claudin 18 isoform 2 in tumours of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer. 2024;27(5):1058-1068. 3. Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133-2141. 4. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med. 2021;11(11):1095. Published 2021 Oct 26. 5. Rohde C, Yamaguchi R, Mukhina S, et al. Comparison of claudin 18.2 expression in primary tumours and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870-876. 6. Angerilli V, Callegarin M, Govoni I, et al. Heterogeneity of predictive biomarker expression in gastric and esophago-gastric junction carcinoma with peritoneal dissemination. Gastric Cancer. Published online April 9, 2025. 7. Scheel AH, Penault-Llorca F, Hanna W, et al. Physical basis of the 'magnification rule' for standardized Immunohistochemical scoring of HER2 in breast and gastric cancer. Diagn Pathol. 2018;13(1):19.